Frontier QA Organization: Governing AI Agents in GxP Pharma
Discover how Frontier QA Organizations govern AI agents to automate validation, audits, compliance, and risk monitoring across GxP pharma environments.
share this
1.0. Introduction: What Is a Frontier QA Organization?
The pharmaceutical industry is entering a new operational era where human teams collaborate with an expanding "army of agents" autonomous AI systems that generate documentation, execute validation, monitor environments, orchestrate audits, and predict risks in real time (@Erika Rhinehart, A Case Study: How pharma organizations are moving into long-horizon agentic workflows).
A Frontier QA Organization is the governance model managing this new reality.
Rather than positioning Quality Assurance (QA) as a reactive reviewer of documentation and deviations, the Frontier QA model transforms QA into:
- A governor of intelligent systems
- An orchestrator of autonomous compliance agents
- A risk intelligence command center
- A continuous validation authority
In this model, QA does not manually create, test, review, and audit every artifact. Instead, QA defines guardrails, risk thresholds, and regulatory alignment frameworks while AI agents execute, monitor, and optimize operations at scale.

The Frontier QA Organization: Governing The Army Of Agents In Pharma: We've entered the era of the "frontier firm"—an organization where artificial intelligence (AI) isn't just a tool but the operating system.
This shift does not replace quality professionals. It augments QA into a higher-order governance function capable of overseeing digital labor at enterprise scale while ensuring regulatory rigor.
At xLM, we operationalize this vision through our Army of GxP Agents.
2.0. xLM’s Army of Agents: Enabling the Frontier QA Organization
The Frontier QA Organization requires intelligent execution layers across the entire GxP lifecycle. xLM’s structured agentic architecture delivers this.
Our model builds on five core primitives:
2.1. Autonomous Content & Validation Intelligence
1. URS Agent – Autonomous Requirements Intelligence
The URS Agent generates regulator-aligned requirements compliant with GAMP 5, FDA CSA, Annex 11, and 21 CFR Part 11. It continuously updates requirements as regulations or systems evolve, ensuring living compliance rather than static documentation.
ROI from the agent includes:
- 85–90% reduction in URS documentation effort
- 70% faster validation initiation
- 90% reduction in URS-related rework
- Zero documentation gaps during audits
In a Frontier QA model, QA governs the rules; the URS Agent enforces them.
2. Test Script Agent – Autonomous GxP Test Design
This agent converts requirements and risk controls into structured IQ, OQ, and PQ scripts with built-in traceability. It eliminates subjective interpretation and ensures structured compliance coverage.
ROI from the agent includes:
- 90% reduction in test script creation effort
- 75% faster validation preparation
- 100% requirements-to-test traceability
3. TraceMatrix Agent – End-to-End Compliance Traceability
The TraceMatrix Agent dynamically links requirements, risks, and tests, enabling instant impact assessment during changes and real-time compliance visibility.
ROI from the agent includes:
- 100% traceability coverage
- 90% reduction in audit preparation effort
- Zero missing trace links
In a Frontier QA organization, traceability is a live intelligence layer, not a spreadsheet.

2.2. GxP Agentic Process Automation
1. RPA Agent – Compliance-Embedded Workflow Automation
The RPA Agent automates deviation management, CAPA orchestration, and SOP-driven workflows while maintaining full audit trails.
ROI from the agent includes:
- 70–80% reduction in compliance workload
- 60% faster deviation and CAPA resolution
- Zero manual documentation errors
QA no longer chases paperwork; QA supervises intelligent process execution.
2.3. Autonomous Multi-Platform Validation
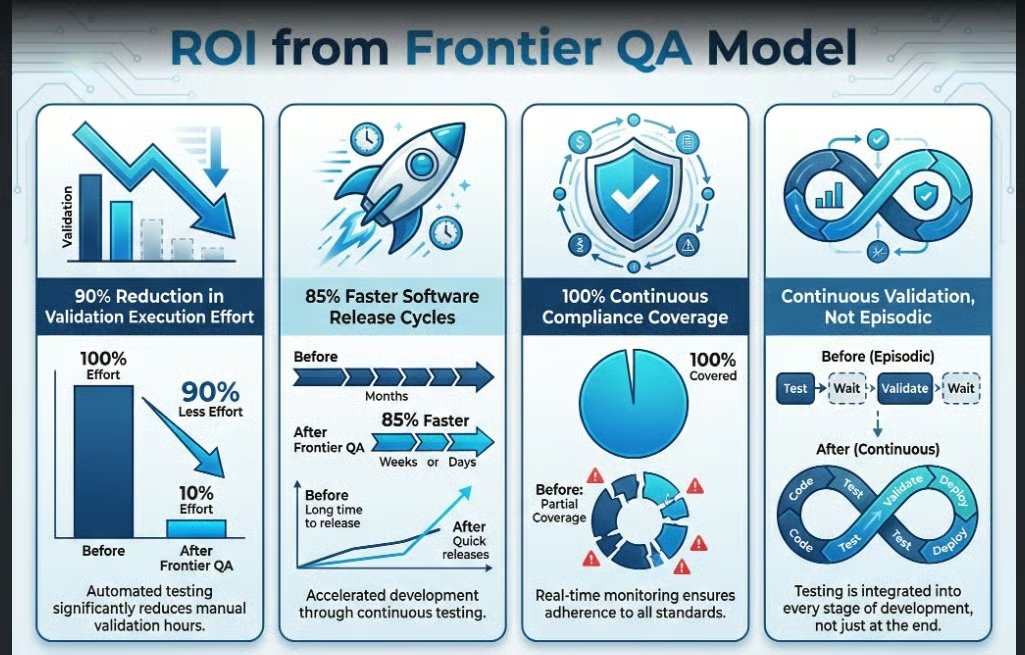
1. GxP Software Validation Agents (Browser | Mobile | Desktop)
These agents execute validation scripts autonomously, capture inspection-ready evidence, and support continuous qualification instead of periodic validation.
ROI from the agent includes:
- 90% reduction in validation execution effort
- 85% faster software release cycles
- 100% continuous compliance coverage
In a Frontier QA model, validation becomes continuous, not episodic.
2.4. Predictive GxP Intelligence
1. Temperature Mapping Agent
Environmental control is critical for GxP compliance across warehouses, cleanrooms, cold chains, and manufacturing facilities. Traditional temperature mapping is manual, time-intensive, and prone to documentation gaps. The Temperature Mapping Agent transforms this process into an autonomous, intelligence-driven capability delivering faster execution, predictive deviation detection, and continuous thermal compliance assurance.
ROI from the agent includes:
- 90% reduction in manual mapping effort
- 75% faster execution cycles
- 60% reduction in thermal deviations
2. Predictive Maintenance Agent
Unplanned equipment failures in regulated manufacturing disrupt production and cause compliance risks, quality issues, and financial loss. The Predictive Maintenance Agent moves maintenance from reactive fixes to data-driven prevention, using advanced analytics to predict failures. It helps pharma firms boost uptime, cut costs, and improve operations.
ROI from the agent includes:
- 40–60% reduction in unplanned downtime
- 30–50% reduction in maintenance costs
- 20–30% increase in OEE
3. Environmental Monitoring Agent
Maintaining controlled environmental conditions ensures product quality, patient safety, and regulatory compliance in GxP facilities. Manual monitoring and fragmented reporting often cause delayed responses and compliance blind spots. The Environmental Monitoring Agent introduces continuous, AI-driven surveillance across critical parameters, delivering real-time visibility, predictive alerts, and automated, inspection-ready reporting.
- 60% reduction in environmental excursions
- 90% automation of monitoring and reporting
- 100% real-time compliance visibility
2.5. Autonomous GxP Vendor Audit
1. GxP Vendor Audit Agent
This agent conducts structured, regulator-grade audits autonomously, validating evidence in real time and generating inspection-ready reports.
ROI from the agent includes:
- 70–85% reduction in audit cycle time
- 60–75% reduction in total audit costs
- 5–10× increase in concurrent audit capacity
- Real-time audit risk scoring
In the Frontier QA framework, audits are continuous intelligence systems, not once-a-year events.
Reengineering GxP Vendor Audits for the AI Era: Reengineer GxP vendor audits with AI-powered continuous intelligence to scale compliance, reduce audit cycles, improve vendor oversight, and ensure readiness.

3.0. How the Frontier QA Organization Increases ROI for Pharma Firms
The frontier QA organization perspective stresses that organizations deploying AI agents must evolve governance to supervise digital labor responsibly. The real value lies in structured orchestration, oversight, and risk governance of autonomous systems.
By adopting the Frontier QA model with xLM’s Army of Agents, pharma firms unlock measurable ROI across five dimensions:
1. Labor Arbitrage to Digital Leverage
Manual GxP documentation, validation execution, mapping, and auditing are labor-intensive and repetitive.
xLM’s agents deliver:
- 70–90% workload reduction across validation and QA processes
- 5–10× audit scalability without headcount expansion
- Massive compression of validation timelines
This converts compliance from a cost center to scalable digital infrastructure.

2. Continuous Compliance = Reduced Regulatory Risk
Traditional QA models are reactive and episodic.
The Frontier QA Organization is:
- Continuous processes running without interruption, ensuring consistent performance.
- Predictive abilities to foresee events using current and past data, enabling proactive decisions.
- Real-time processing offering immediate insights and responses, reducing delay and boosting responsiveness.
- Traceable systems keeping clear, auditable records to ensure transparency and accountability
This reduces inspection findings, eliminates documentation gaps, and strengthens inspection confidence.

3. Faster Release Cycles Without Compliance Compromise
With autonomous validation and continuous qualification:
- Software releases accelerate by up to 85%
- Validation costs drop by up to 70%
- Compliance coverage improves to 100% traceability
Speed and compliance are no longer trade-offs.

4. Proactive Risk Management
Predictive maintenance, environmental intelligence, and real-time audit scoring shift the organization from:
- Deviation Management → Risk Prevention
- Periodic Audit → Continuous Assurance
- Manual Review → AI-Driven Oversight
This directly reduces product loss, recalls, and quality events.

5. Strategic Repositioning of QA
The most significant ROI is organizational.
In the Frontier QA Organization:
QA becomes:
- AI Governance Authority
- Risk Intelligence Hub
- Digital Compliance Architect
- Enterprise Trust Custodian
Instead of expanding teams linearly, firms scale compliance exponentially through supervised autonomy.

4.0. The Future of Validation Is Frontier
Pharma is not simply adopting AI. It is entering an era where intelligent agents perform regulated work at scale.
The question is no longer: “Can AI help with validation?”
The real question is: “Who governs the army of agents?”
The Frontier QA Organization is the answer.
At xLM – Continuous Intelligence, we do not just deploy agents. We architect the governance model ensuring they operate:
- Transparently
- Traceably
- Audit-ready
- Risk-aligned
- Regulator-confident
The next competitive advantage in pharma will not be automation alone. It will be governed autonomy.
Firms adopting the Frontier QA model early will define the next decade of compliant digital transformation.
share this

