AI in GxP: Insights from AstraZeneca’s Digital Strategy Head
Our LinkedIn Live on AI in GxP drew 600+ registrations and strong engagement, highlighting real-world perspectives from pharma IT, manufacturing, and compliance
share this

1.0. Introduction: A Signal, Not a Spike
Our recent LinkedIn Live, “GxP in the Age of AI: Real-World Perspectives from Pharma IT and Manufacturing,” resonated beyond expectations.
The session attracted 624 registrations, all organic. This led to 393 unique viewers and a healthy engagement rate for a technical, unscripted discussion on AI in GxP environments.
For a technical, unscripted discussion on AI and regulated manufacturing, these numbers indicate: The industry no longer asks if AI belongs in GxP but how.
Hosted by xLM Continuous Intelligence, the conversation featured Mr. Bob Buhlmann, a pharma leader with over 30 years in consulting and senior roles at Amgen, now leading Digital Quality and Strategy at AstraZeneca.
This was not a theory-heavy talk. The focus was execution, what works, where organizations struggle, and how to stay inspection-ready while moving fast.
2.0. AI in GxP Manufacturing: From Curiosity to Capability
The conversation began with a key question: How is AI impacting GxP manufacturing today?
The consensus was clear: AI is present but adoption varies. Advanced analytics and automation grow common, but trust and intended use remain decisive. Technology is no longer the main limit; governance and clarity are.
A key distinction emerged:
- AI as decision support is gaining traction.
- AI as decision maker remains a regulatory red line.
This framing set the tone for the discussion.
3.0. Trust, Validation, and the Reality of “Validated AI”
A pressing concern was whether AI systems, especially GenAI, can be trusted in validated environments.
The discussion highlighted an important nuance:
- Validation does not require determinism
- It requires controlled intended use, transparency, and evidence
AI systems fit validated frameworks if:
- Their scope is clear
- Outputs are reviewed by humans
- Audit trails are complete and explainable
This mirrors existing practices, like junior engineers drafting URS or test cases that SMEs review and approve.
Related LinkedIn Chatter: AI VALIDATION ISN'T ARCHITECTURE. IT'S MEDICINE.
4.0. AI Agents in Core GxP Processes: Hype vs Reality
The discussion moved to a practical segment: AI agents embedded in GxP workflows.
Examples included:
- Validation documentation generation (URS, Test Scripts, Trace Matrices)
- CAPA analysis
- Batch record review
- Risk assessments (FMEA)
The key insight: Value lies not in replacing humans but in restructuring workflows around agentic capabilities.
When AI agents act as accelerators with humans in the loop, organizations see:
- 10× timeline compression
- Sub-3-month ROI
- Improved consistency and auditability
Related LinkedIn Chatter:
- Stop Blaming AI Models. Your Test Data Is Failing You.
- #093: Continuous Intelligent Validation (cIV): From Months of Manual Validation to Minutes of Intelligent Execution
- 𝗰𝗧𝗠: 𝗧𝗵𝗲 𝗡𝗲𝘅𝘁 𝗚𝗲𝗻𝗲𝗿𝗮𝘁𝗶𝗼𝗻 𝗼𝗳 𝗧𝗲𝗺𝗽𝗲𝗿𝗮𝘁𝘂𝗿𝗲 𝗠𝗮𝗽𝗽𝗶𝗻𝗴 : 𝗔𝗿𝗲 𝘆𝗼𝘂 𝘀𝘁𝗶𝗹𝗹 𝗿𝗲𝗹𝘆𝗶𝗻𝗴 𝗼𝗻 𝗺𝗮𝗻𝘂𝗮𝗹, 𝗲𝗿𝗿𝗼𝗿-𝗽𝗿𝗼𝗻𝗲 𝘁𝗲𝗺𝗽𝗲𝗿𝗮𝘁𝘂𝗿𝗲 𝗺𝗮𝗽𝗽𝗶𝗻𝗴 𝗽𝗿𝗼𝗰𝗲𝘀𝘀𝗲𝘀 𝘁𝗵𝗮𝘁 𝗱𝗿𝗮𝗶𝗻 𝘁𝗶𝗺𝗲 𝗮𝗻𝗱 𝗿𝗲𝘀𝗼𝘂𝗿𝗰𝗲𝘀—𝗮𝗻𝗱 𝗽𝘂𝘁 𝗿𝗲𝗴𝘂𝗹𝗮𝘁𝗼𝗿𝘆 𝗰𝗼𝗺𝗽𝗹𝗶𝗮𝗻𝗰𝗲 𝗮𝘁 𝗿𝗶𝘀𝗸?
5.0. Regulators and Inspections: What Agencies Actually Ask
A recurring theme was regulatory engagement.
Contrary to fears, inspectors do not reject AI outright. They ask:
- What is the intended use?
- Where are decisions made?
- What evidence supports the output?
- How is risk controlled?
Unintended risk often arises not from AI itself but from how organizations describe it. Over-promising autonomy or vague language can create inspection signals.
The recommendation was simple:
“Be precise. Be boring. Be evidence-driven”
Related LinkedIn Chatter: From AI pilots to regulated scale: a blueprint for Pharma leaders
6.0. Inspection Readiness for AI-Enabled Workflows
Inspection readiness in AI-enabled environments looks familiar but deeper.
What matters most:
- Clear process boundaries
- Documented human oversight
- Robust audit trails
- Traceability from input → output → approval
In some cases, AI-enabled systems improve inspection readiness by generating richer, more consistent evidence than manual processes.
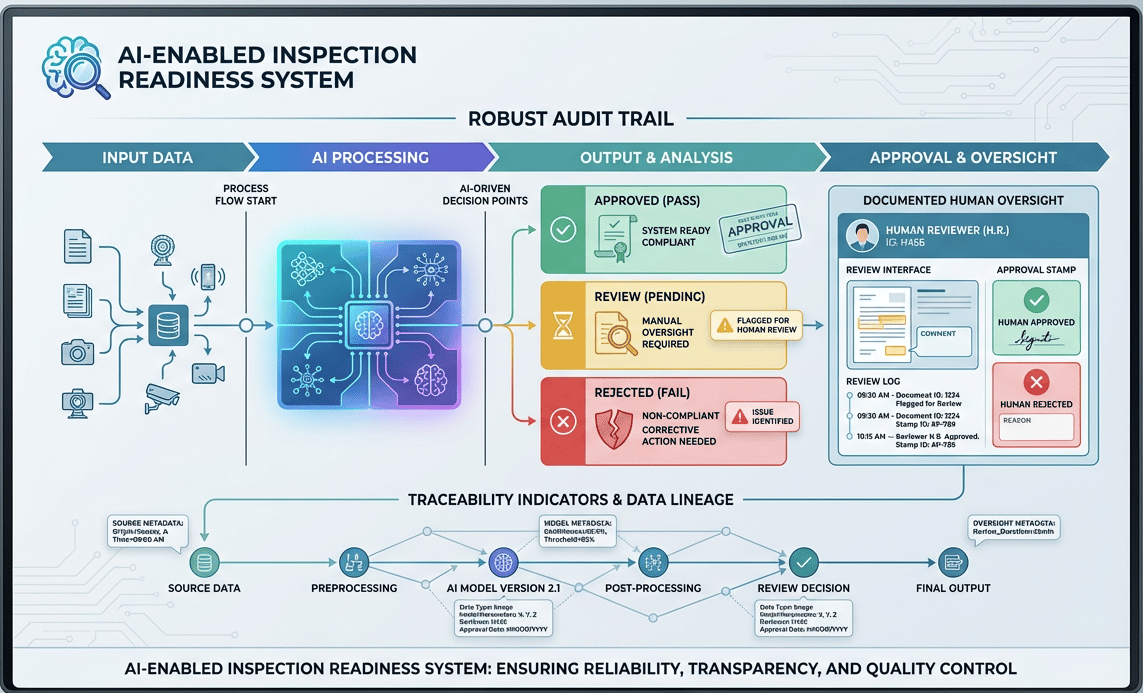
Related LinkedIn Chatter - As AI plays a greater role in manufacturing, auditors will increasingly focus on evaluating these AI systems for compliance.
7.0. Real-World Agentic Use Cases: Beyond Slides and Demos
Several concrete examples showed what “pragmatic AI” looks like:
- Autonomous validation execution with xLM’s cIV, including audit trails, execution logs, and session recordings
- Plug-and-play temperature mapping with xLM’s cTM and environmental monitoring with xLM’s cEMS using IIoT sensors with real-time analytics and anomaly prediction
- Regulatory impact analysis, where new regulations trigger automated SOP reviews and redlining
- Automated FMEA drafting, accelerating early-stage risk analysis
The takeaway was striking: If organizations reimagine processes instead of automating old ones, efficiency gains of 10–20× are realistic.
Related LinkedIn Chatter: #093: Continuous Intelligent Validation (cIV): From Months of Manual Validation to Minutes of Intelligent Execution
8.0. Leadership, Governance, and Organizational Readiness
AI adoption cannot be delegated to IT or QA alone.
Successful implementations share three traits:
- Top-down sponsorship
- Clear goals and roadmaps
- Governance that enables, not suffocates
Strong quality governance means better boundaries, not more paperwork.
Winning over skeptical stakeholders often starts with one thing:
“Show them working evidence, not strategy decks”
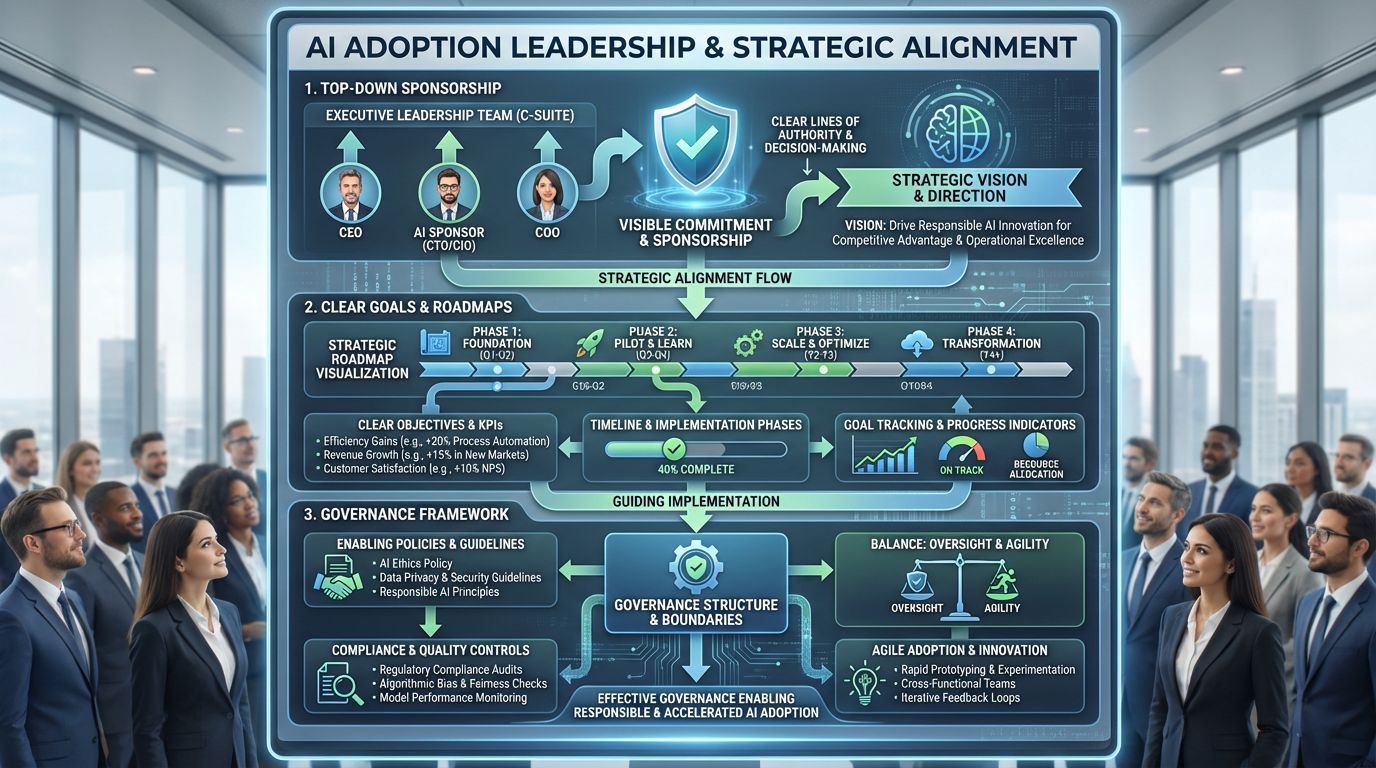
Related LinkedIn Chatter: #081: The GxP AI leadership framework: Align → Activate → Amplify → Accelerate → Comply (A4C)
9.0. Frontier Organizations: What’s Coming Next
The conversation closed by looking ahead.
A compelling vision emerged: Frontier GxP organizations powered by layers of AI agents working continuously, supervised by both:
- Agentic QA layers
- Human experts
Such organizations could operate 24×7, maintain continuous compliance, and compress validation timelines from months to minutes.
The technology exists.
The real question is organizational courage.
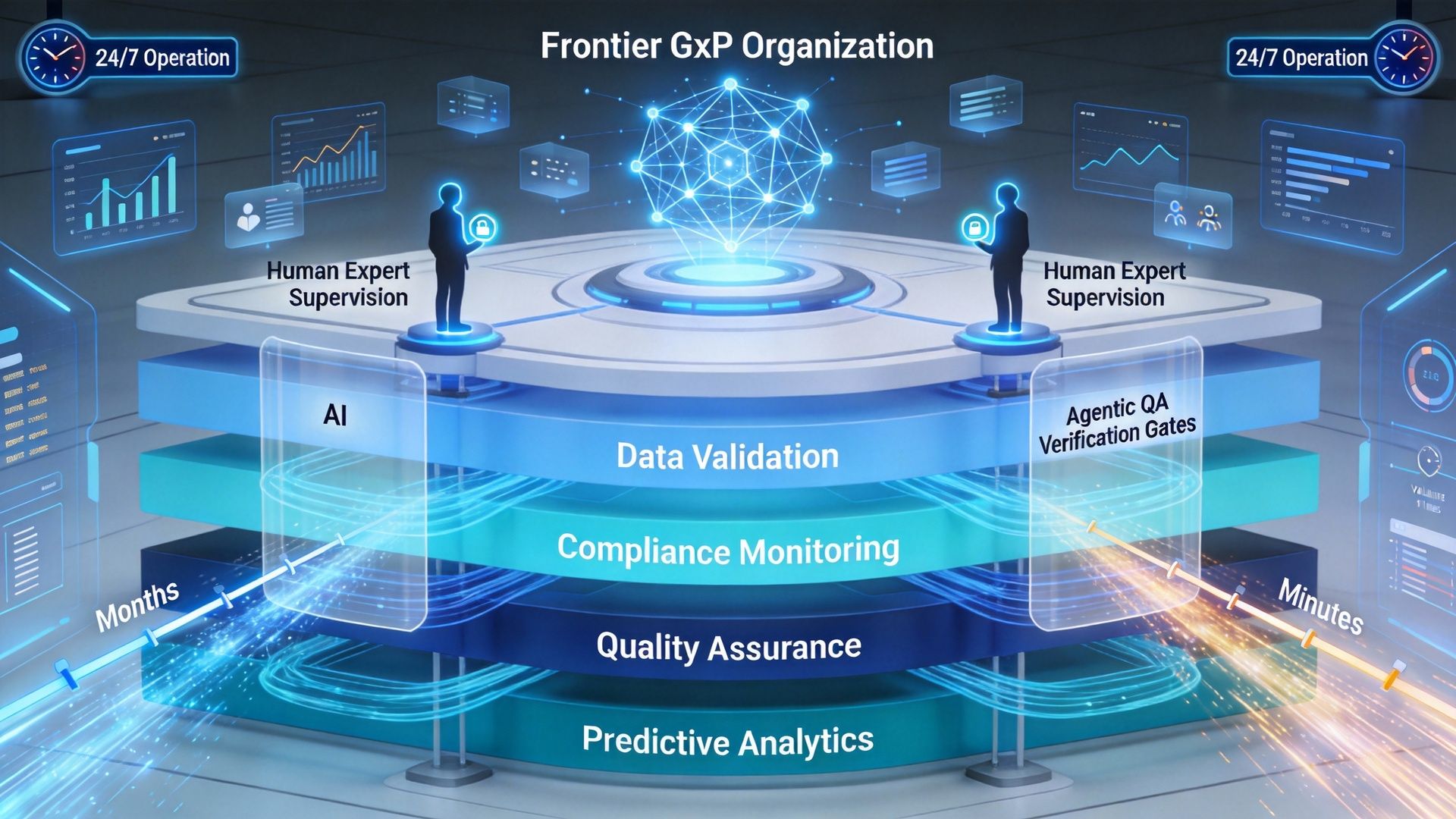
Related LinkedIn Chatter: How Pharma Can Transform Compliance? The Frontier Software Validation Organization Brings a Competitive Advantage.
10.0. Conclusion: Moving Fast Without Losing Control
If there was one message from this session, it was:
“You can move fast in GxP with AI but only if you are clear on intended use, governance boundaries, and inspection-ready evidence”
AI is no longer a future topic.
It is a present execution challenge and opportunity.
The winning organizations will not have the flashiest models but will combine pragmatism, quality discipline, and intelligent automation.
share this

